Why Manufacturers Should Consider Nitrogen Dioxide Sterilization
Nitrogen dioxide sterilization is superior to EtO for sterilizing the external surfaces of drug and biologic combination products.
Driven by the need to improve patient safety, reduce production costs, and optimize efficiency, new drugs, technologies, materials, and processes are continually being introduced. For example, new device and delivery system designs such as dual-chamber prefilled syringes and drug-device combination products offer increased functionality while simplifying medical procedures. But there’s a tradeoff: Along with new designs and applications, new sterilization challenges are arising. Because prefilled syringes and drug-delivery systems are frequently used in the operating room, their exterior surfaces must be sterilized, even when the drug itself is aseptically filled.
The most common methods used for sterilizing medical devices include ethylene oxide (EtO) and gamma radiation. While these methods have been used for decades and are known to be effective, the use of nitrogen dioxide (NO2) gas offers product designers greater freedom in material selection while enabling drug and biologics manufacturers to provide sterile primary packaging for their products. NO2 sterilization is advantageous for a number of reasons: It is a rapid, room-temperature process that can be performed without deep vacuum, it does not readily penetrate container closure systems and device materials, and it can be performed in-house, promoting increased efficiency and cost savings.
How NO2 Sterilization Works
The NO2 sterilization process is carried out in specially designed sterilization chambers that are available in load volumes from 360 to 5000 L. In general, the NO2 process is similar to that used in most other gas sterilization processes. First, the chamber is evacuated to a specified pressure, following which the sterilant and humidity are introduced. During the dwell period, the medical devices are sterilized, and then the sterilant and humidity are removed. While NO2 gas and humidity are typically introduced and removed using vacuum, a deep vacuum is not required. Multiple injections and dwells can be used to achieve the specified sterility assurance level (SAL).
The sterilization chamber requires minimal utilities. The NO2 cylinders are contained within the sterilizer, as is the water reservoir. An on-board scrubber system contains a chemisorbant material to remove the NO2 from the exhaust gas stream, and the resulting waste material is solid and nonhazardous. The chamber is also equipped with outlets to connect it to a plant ventilation system. In addition, the unit’s control system incorporates multiple electrochemical NO2 sensors to alert operators to potentially unsafe conditions in and around the enclosure.
The biological indicator (BI) organism used in the NO2 sterilization process is the spore of Geobacillus stearothermophilus, the traditional indicator organism used in both steam and hydrogen peroxide sterilization methods. The process used to sterilize a defined product is validated to ISO 14937:2009, a general sterilization standard that can be applied to novel, nontraditional sterilization processes. ISO 14937:2009 is similar to ISO 11135:2014, which governs EtO sterilization.
To validate a process, it is advisable to use the ‘overkill’ approach described in Annex D of ISO 14937:2009, which enables manufacturers to define a sterilization process in which a minimum six-log reduction in the BI population is achieved during a half cycle. The specified process consists of two half cycles, which are validated to demonstrate a repeatable SAL of 10–6. Provided that a justification can be provided showing that a given device is representative of a product family, manufacturers need only validate a single sterilization process for the entire family.
Advantages of NO2 Sterilization
Room-Temperature Process. Because the EtO sterilization process is performed at elevated temperatures, it is not well suited for sterilizing devices containing new drugs and biologics, which exhibit limited stability at elevated temperatures. In contrast, because NO2 gas sterilization is performed at room temperature, it can be used to sterilize drug- and biologic-delivery devices, especially prefilled syringes developed for use in the sterile field of the operating room. While the contents of prefilled syringes may have been aseptically processed, sterilizing the syringes’ exterior surfaces can enhance patient safety during surgery. As shown in Table I, a room-temperature sterilization process reduces the risk that biologics will become agglomerated or denatured and that drugs will degrade.
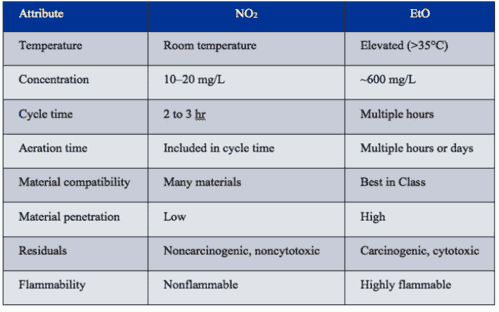
Table I: Comparison of the key attributes of NO2 and EtO sterilization processes.
The NO2 sterilization process can be accelerated by using a deep vacuum level of P < 100 mm Hg to drive the sterilant into the packaging and device geometries, as presented in Figure 1. However, a minimal vacuum process has also been developed for pressure-sensitive products, as shown in Figure 2.
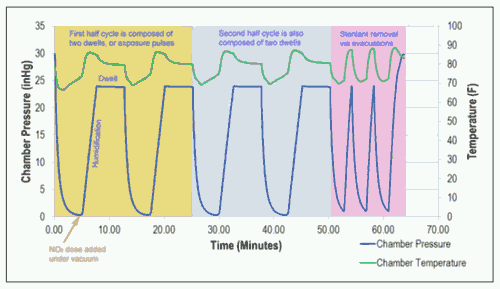
Figure 1: The temperature and pressure profiles of a typical NO2 vacuum sterilization cycle.
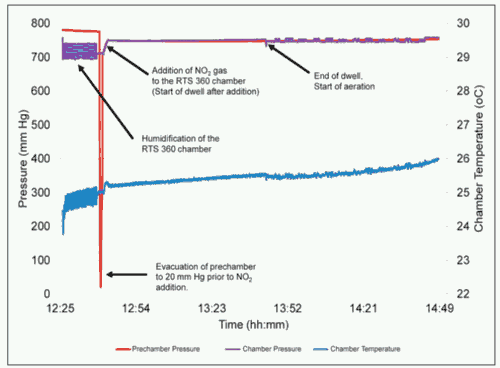
Figure 2: The temperature and pressure profiles of a typical NO2 minimal-vacuum sterilization cycle.
One such application is a prefilled syringe, in which the expansion of air in the headspace can cause the stopper to move under vacuum. To minimize the risk of stopper movement and the ingress of sterilant into the syringe, a vacuum level of approximately 90% of sea level pressure (P > 700 mm Hg) is employed to help humidify the chamber and facilitate NO2 dosing. This pressure level is equivalent to an elevation of 700 m. Both water vapor and NO2 gas readily diffuse through the porous packaging, typically a Tyvek lid on a blister tray, sterilizing the surfaces of the syringe. Under minimal vacuum, aeration is also performed by continuously exchanging the chamber environment with filtered room air.
Rapid Sterilization. Faster than EtO gas sterilization, a typical NO2 sterilization vacuum cycle lasts approximately two hours. In contrast, the NO2 sterilization process under minimal vacuum typically lasts approximately three hours because the diffusion of NO2 and water vapor is not aided by a vacuum to force the gases past the sterile barrier packaging. Both of these process time estimates include an aeration step, which is carried out in the sterilization chamber rather than in a special aeration room. Because the actual duration of a sterilization cycle depends on the device’s geometry, materials, and packaging, it can be determined only on the basis of the validation process.
A Safe Process. In addition to being a low-temperature and rapid process, NO2 sterilization is unlikely to affect the contents of prefilled syringes. At room temperature, NO2 does not readily diffuse past most stopper materials. It remains primarily on the syringe surface, from which it can be readily removed using aeration. In contrast, EtO sterilization is known to penetrate stopper materials over time, increasing the risk that residuals will migrate into the drugs or biologics and posing a safety concern. Radiation can also have a negative impact on the contents of prefilled syringes, causing them to degrade or agglomerate.
The European Pharmacopoeia (EP) specifies that water for injection (WFI) have a nitrate limit of 0.2 ppm. Recent studies involving parenteral drug containers filled with WFI showed that the level of NO2 ingress was below the 0.1-ppm detection limit for nitrates, as measured by poststerilization ion chromatography tests. This result indicates that prefilled syringes exposed to NO2 meet the EP standard for nitrate levels. While the WFI data do not preclude the need to perform quality and safety testing on the actual drug or device, they can serve to complement quality and safety testing.
In the case of implantable medical devices, NO2 sterilization leaves behind low concentrations of sterilant residuals. In addition, recent device testing has demonstrated that on compatible metals and polymers, these residuals are noncytotoxic, nonirritating, and nonsensitizing, even when they appear on devices used in mucosal membrane applications. Nevertheless, even devices composed of materials of known compatibility must be tested during the validation process.
Conclusion
The growing availability of biopharmaceuticals—coupled with the prevalence of increasingly complex drug-delivery and implantable products—makes it incumbent on medical device manufacturers to select an appropriate sterilization process. Additionally, cost-reduction efforts and market responsiveness are driving shorter sterilization turnaround times and reduced time to market, further challenging device designers.
While such traditional sterilization methods as EtO and gamma radiation continue to serve the medical device industry, manufacturers of sensitive products—including novel drugs, technologies, and materials—now have another sterilization option. NO2 sterilization, in addition to its processing and safety advantages, can also benefit device makers interested in improving manufacturing efficiency.
Evan Goulet, PhD, is director, sterilization operations at Baltimore-based Noxilizer Inc.
From mddionline (Knowledge Network), sharing information on Noxilizer Inc, a provider of sterilization equipment
http://www.mddionline.com/article/why-manufacturers-should-consider-nitrogen-dioxide-sterilization-04-23-15